991 | Add to Reading ListSource URL: ec.europa.euLanguage: English - Date: 2008-12-04 08:53:46
|
|---|
992![PROGRAMA ESTÁGIO PÓS-DOUTORAL SANOFI 2013 EDITAL Nº [removed]Processo[removed][removed] PROGRAMA ESTÁGIO PÓS-DOUTORAL SANOFI 2013 EDITAL Nº [removed]Processo[removed][removed]](https://www.pdfsearch.io/img/ecaa64ef7d8a3127edb5682ace622a42.jpg) | Add to Reading ListSource URL: www.capes.gov.br- Date: 2014-02-14 10:41:13
|
|---|
993![KENYA INDUSTRIAL PROPERTY INSTITUTE INDUSTRIAL PROPERTY JOURNAL (Journal of Patents, Industrial Designs, Utility Models and Trade marks) No[removed] KENYA INDUSTRIAL PROPERTY INSTITUTE INDUSTRIAL PROPERTY JOURNAL (Journal of Patents, Industrial Designs, Utility Models and Trade marks) No[removed]](https://www.pdfsearch.io/img/20f4be113054f33aa2894eadde920ee0.jpg) | Add to Reading ListSource URL: www.kipi.go.keLanguage: English - Date: 2014-01-16 01:03:36
|
|---|
994 | Add to Reading ListSource URL: en.sanofi.comLanguage: English - Date: 2014-06-20 12:38:41
|
|---|
995 | Add to Reading ListSource URL: en.sanofi.comLanguage: English - Date: 2014-05-07 11:04:13
|
|---|
996 | Add to Reading ListSource URL: en.sanofi.comLanguage: English - Date: 2014-06-24 04:44:12
|
|---|
997 | Add to Reading ListSource URL: en.sanofi.comLanguage: English - Date: 2014-07-08 00:57:36
|
|---|
998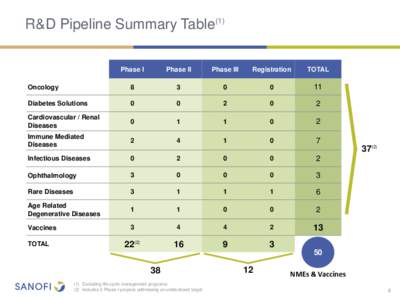 | Add to Reading ListSource URL: en.sanofi.com- Date: 2014-07-22 08:14:57
|
|---|
999 | Add to Reading ListSource URL: en.sanofi.comLanguage: English - Date: 2014-06-16 11:21:43
|
|---|
1000![[removed]to that. My own comments were meant to be a [removed]to that. My own comments were meant to be a](https://www.pdfsearch.io/img/37b1cbe3cb377d404cca0a9a4e7f2be3.jpg) | Add to Reading ListSource URL: www.fda.govLanguage: English - Date: 2009-01-05 15:12:35
|
|---|