| Document Date: 2013-08-14 05:47:58
Open Document File Size: 246,07 KBShare Result on Facebook
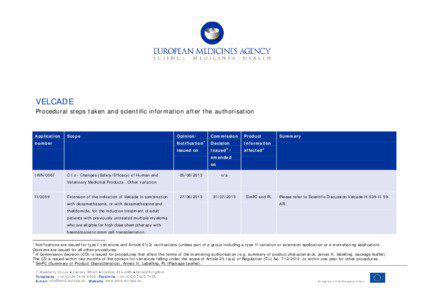
City Bedford / Velcade / London / / Company Ben Venue Laboratories / / Country United States / Croatia / United Kingdom / / / Event FDA Phase / Product Issues / / Facility Canary Wharf / / IndustryTerm above mentioned medicinal product / finished product / pharmaceutical forms / medicinal product / manufacturing sites / manufacturing site / manufacturing / immunological medicinal products / sterile medicinal products / manufacturing process / / MedicalCondition multiple myeloma / previously treated light-chain amyloidosis / solid tumors / thrombocytopenia / amyloidosis / significant modifications dysfunction / peripheral neuropathy / AL amyloidosis VELCADE C.I / previously untreated multiple myeloma / hepatic dysfunction / virus infection / Progressive Multifocal Leukoencephalopathy / constipation / optic neuropathy / blindness / / MedicalTreatment retreatment / immunosuppressive therapy / chemotherapy / bone marrow transplant / / OperatingSystem Microsoft Vista / / Organization European Commission / European Union / E-mail info@ema.europa.eu Website www.ema.europa.eu An / / Person John Cunningham / / / Position specialist / / Product phenytoin / VELCADE / dexamethasone / carbamazepine / prednisone / CAN-2007 / MMY-3002 / hepatic function / / ProvinceOrState Ohio / / Technology pharmacokinetics / chemotherapy / transplantation / / URL www.ema.europa.eu / /
SocialTag |

